Research
Published 9 September 2021Advanced Alloy Anodes for Next-generation Rechargeable Batteries
Batteries help power modern life. Advances in battery technology have led to increasing levels of energy storage and power output however we are at technical, economic, and safety crossroads....
Our current workhorse battery, the lithium-ion battery, isn’t suitable for next-generation battery applications like transport (electric cars and planes) and large-scale energy storage (the NZ power grid). Magnesium (Mg) rechargeable batteries show many advantages compared to lithium-ion batteries, including the high elemental abundance of Mg in the earth’s crust (Figure 1a), lower production costs, and higher levels of energy storage per unit volume (higher theoretical volumetric energy density; Figure 1b). Mg rechargeable batteries are still a new player on the battery scene and so to truly unlock their next-generation potential more fundamental research is required. The main challenge to overcome with Mg rechargeable batteries is the poor electrochemical performance of commercial magnesium anodes (the negative terminal of the battery). This Marsden Funded Fast-Start research project looks to overcome this challenge.
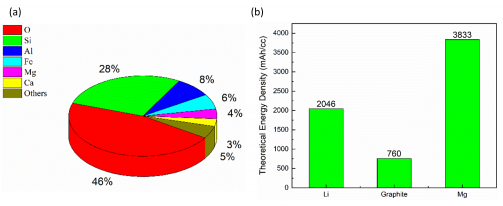
Figure 1 (a) Elemental abundance of various elements in the earth’s crust. Mg is the world’s 3rd most abundant metal (magenta). (b) Mg metal has higher theoretical volumetric energy density than Li metal anodes, and conventional graphite (LiC6) anodes (Supplied).
The project aims to open a novel route for developing better Mg rechargeable batteries by investigating new anodes where Mg is in combination with other metals (alloys) including those with a specific order or structure (intermetallic phases). The Principal Investigator Dr. Shanghai Wei (Figure 2) works collaboratively with Professor Mark Taylor, Professor Wei Gao, Associate Professor Peng Cao and Associate Prof Tilo Söhnel, leading a team of PhD students at the University of Auckland, to understand the electrochemical charge/discharge behaviour of different Mg alloys with controlled microstructure.

Figure 2: The research team working on this Fast-Start project. From left to right: Fanglei Tong (PhD student), Xize Chen (PhD student), Tony Wang (PhD student), Dr Shanghai Wei (PI, University of Auckland), Professor Wei Gao (University of Auckland), Kirill Panov (PhD student), Associate Professor Peng Cao (University of Auckland), Associate Professor Tilo Söhnel (University of Auckland), Ryan Silk (PhD student). (Supplied).
In the last two years, this research team has designed and studied a series of Mg alloys as anode materials for magnesium rechargeable batteries. The team have investigated how these different Mg alloy anodes behave when used in a battery. They have found that magnesium-tin (Mg-Sn) alloys show an obvious higher voltage plateau than pure Mg. A higher voltage plateau could translate to a ‘better’ battery as a higher voltage is consistently produced across a battery’s operating lifetime and conditions.
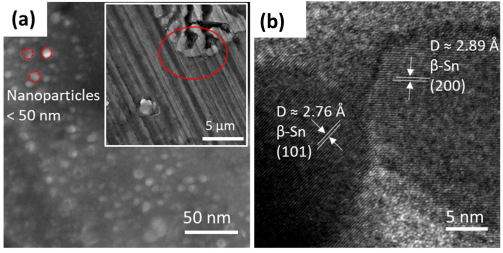
Figure 3 (a) Scanning electron microscopy (SEM) image and (b) high-resolution transmission electron microscopy (HRTEM) image of individual Sn nanoparticles on the surface of Mg-Sn alloy anode after a 1-hour discharge process (current density of 0.05 mA/cm2). (Supplied).
To gain insight into how the Mg-Sn alloys make better batteries, the researchers are using a variety of electron microscopy techniques to observe atomic changes on the battery surface. For example, scanning and transmission electron microscopy images of the Mg-Sn alloy surface show small, nano-sized Sn particles post battery discharge (Figures 3a and b). Through further analysis they have found that these Sn particles play an important role in the charge/discharge cycle by converting Mg back into its usable form. The combination of alloy fabrication, electrochemical measurements, and microscopy characterization allows the team to steadily improve battery design and theory.
Overall, the team is designing and synthesising high energy density metallic anodes, promoting a third breakthrough toward development of practical rechargeable Mg batteries.
Additional information:
The research article describing the main findings of this research will be found here once it has been published.
A freely available review article by the team describing magnesium alloys as anodes can be found here.
If you are interested in following up with any of the content discussed here please contact the lead investigator, Dr Shanghai Wei, by email (s.wei@auckland.ac.nz ).
RESEARCHER
Dr Shanghai Wei
ORGANISATION
University of Auckland
FUNDING SUPPORT
Marsden Fund
CONTRACT OR PROJECT ID
UOA1817
